TECHNICAL BRIEF NO. 18 2007
PDF version
Knowledge Translation at the Canadian Institutes of Health Research: A Primer
Jacqueline Tetroe, MA
Senior Policy Analyst, Knowledge Translation Portfolio
About the Canadian Institutes of Health Research
The Canadian Institutes of Health Research (CIHR) is the major federal agency responsible for funding health research in Canada. It aims to excel in the creation of new health knowledge and to translate that knowledge from the research setting into real-world applications. The results are improved health for Canadians, more effective health services and products, and a strengthened Canadian health-care system. It was created under an act of parliament that came into force on June 7, 2000.
CIHR consists of 13 "virtual" institutes, each headed by a scientific director and assisted by an institute advisory board. The institutes work together to shape a national health research agenda for Canada. They bring together researchers, health professionals and policymakers from voluntary health organizations, provincial government agencies, international research organizations, industry, and patient groups from across the country—all of whom share an interest in improving the health of Canadians.
The work of the institutes embraces the four pillars of health research: (1) biomedical; (2) clinical; (3) health systems and services; and (4) population and public health. A major challenge for the institutes is to forge relationships across disciplines to stimulate integrative, multifaceted research agendas that respond to society's health priorities while adhering to the highest ethical standards. Translating knowledge from the research setting into realworld applications for the benefit of Canadians is a key component of CIHR's mandate and is the topic of this issue of Focus.
Knowledge Translation at CIHR: Definitions, Purpose, and Rationale for Knowledge Translation
CIHR's definition of knowledge translation (KT) has been cited and adapted widely. It reads as follows:
Knowledge translation is the exchange, synthesis and ethically-sound application of knowledge—within a complex system of interactions among researchers and users—to accelerate the capture of the benefits of research for Canadians through improved health, more effective services and products, and a strengthened health care system.
However, when Ian Graham accepted his position as CIHR's vice president of knowledge translation he saw the need to tinker with the definition slightly in order to clarify its critical components. The revised working definition of knowledge translation is
a dynamic and iterative process that includes the synthesis, dissemination, exchange and ethically sound application of knowledge to improve the health of Canadians, provide more effective health services and products and strengthen the healthcare system.
By specifying the importance of synthesis and the ethically sound application of knowledge, the definition also implies that some thought should be given to what knowledge should be translated—and to which audience—keeping in mind how the knowledge could be used. An important implication of this is that while we encourage researchers to translate the results of their studies, they, at the same time, need to be thoughtful about their message and who the appropriate audience is for this message.
Context for the CIHR Definition
As described in the CIHR Act, knowledge translation is a broad concept. It encompasses all steps between the creation of new knowledge and its application to yield beneficial outcomes for society. The concept includes knowledge dissemination, technology transfer, consideration of the ethical context, knowledge management, knowledge utilization, the two-way exchange between researchers and those who apply knowledge, implementation research, technology assessment, synthesis of results within a global context, and the development of consensus guidelines. The overriding principle is that interactions between researchers and stakeholders may vary in intensity, complexity, and level of engagement depending on the nature of the research results and on the needs of the particular stakeholder.
Terms such as continuing medical education (CME), continuing professional development, and translational research have often been used interchangeably with the term knowledge translation, but we would consider each of these to be a subset of KT. CME generally refers to planned educational activities intended to further the education and training of specific health professionals for the enhancement of practice, education, administration, and research—in other words, professional development. The term continuing professional development refers to educational methods beyond the didactic; embodies concepts of self-directed learning and personal development; and considers organizational and system factors. Translational research is about finding solutions to clinical problems. Ideally it involves two-way interactions between basic/fundamental scientists and clinicians and requires moving between scientific discoveries and clinical applications. Translational research stops short of widespread dissemination of the clinical application once it has been proved beneficial by clinical research.
So why are we, at CIHR, so interested in the concept and process of knowledge translation? The facile response to this question is that it is part of our mandate. But why did the parliamentarians who crafted the act that created CIHR include this component? There are two primary reasons. First, the creation of new knowledge often does not, by itself, lead to its widespread adoption or impact health. Second, the past 10 or 15 years have seen increased emphasis on research governance and accountability from the federal and provincial governments, as well as from the public. All of these interested parties want to see the benefits reaped by the taxpayers' dollars invested in health research by moving research into practice/action.
How Widely Can the Concept of Knowledge Translation Be Applied?
KT is an issue at the local, national, and international level. What distinguishes the levels is the end user/ target audience, but the process is essentially the same at each level, and the impact of KT can filter up or down the levels. Consideration of the potential research user is equally important for both basic and applied scientists working within any of CIHR's four pillars of health research. The concept of KT is not at all unique to medical research. It has been used in many other disciplines, where it is known by other names: technology transfer, knowledge management, or change management, to name a few. The process that is KT is appropriate to any discipline—it is about facilitating the uptake of research. While the process is universal, the content/context varies.
But What Does KT Really Mean?
The terms KT and research utilization are often used interchangeably, but we would argue that there are subtle but important differences. The differences are in how one defines "research" and "knowledge." In our view, research is a subset of knowledge. However, for an interesting discussion on the nature of evidence, knowledge, and research, see the work of Jonathan Lomas and others at the Canadian Health Services Research Foundation (Lomas, Culyer, McCutcheon, & McAuley, 2005).
Research utilization has been the term used since the early 1970s to describe the incorporation of research evidence into clinical practice. It is a term used predominantly by the nursing profession, and the definition has become more precise over time. For example, Titler (1999) defined it as a process of using findings from conducting research to guide practice; Brown (1999) said it was the process by which scientifically produced knowledge is transferred to practice; and Estabrooks (1998) said it is the use of research findings in any and all aspects of one's work as a registered nurse. In 2003, Estabrooks (2003) revised her definition to the process by which specific research-based knowledge (science) is implemented in practice. Finally, in 2006, Estabrooks (2006) wrote that research utilization is a specific kind of knowledge utilization whereby the knowledge has a research base to substantiate it. It is a complex process in which knowledge, in the form of research, is transformed from the findings of one or more studies into instrumental, conceptual, or persuasive utilization.
There are many other terms in use in the literature besides knowledge translation and research utilization, such as evidence-based practice; implementation; knowledge mobilization; moving knowledge to practice; knowledge to action; impact; linkage, and exchange; and knowledge transfer. The specific words used are not important per se—what is important is how these terms are operationalized. Ian Graham, CIHR's vice president of knowledge translation, uses the term knowledge to action (Graham, Logan, Harrison, Straus, Tetroe, Caswell, & Robinson, 2006).
Knowledge to Action
Knowledge to action is an organic process with defined steps—a process (see Figure 1).
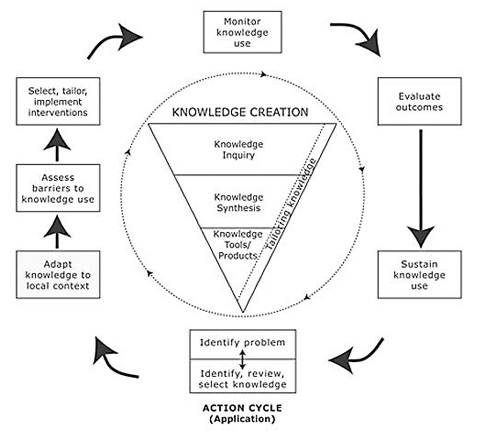
Figure 1. The Knowledge-to-Action Process
(Reprinted with permission from the Journal of Continuing Education in the Health Professions, Vol. 26, No. 1, Graham, I. D. et al., Lost in knowledge translation: Time for a map, pp. 13–24, copyright © 2006, John Wiley & Sons, Inc.)
A full discussion of the model illustrated in Figure 1 can be found in the Graham et al. (2006) article, but there are a couple of features that are worthy of note. First, the idea of a knowledge creation "funnel" conveys the idea that knowledge needs to be increasingly distilled before it is ready for application. The model stressed the importance of synthesis to contextualize and integrate the findings of an individual research study within a larger body of literature. Synthesis can use quantitative or qualitative methods and may take many forms: a literature review; a systematic review following the methods developed by the Cochrane Collaboration; a realist review; a consensus conference or the results from an expert panel. Synthesis is important to be able to create knowledge tools (i.e., provide the data content for incorporation in practice guidelines); it can be used to determine best practice (that needs to be implemented) and to create a context for and establish an evidence base for the knowledge to be translated. Furthermore, it is important to consider and report on the types of evidence used in a synthesis—it establishes the credibility and generalizability of the evidence base of the knowledge to be "translated" (Figure 1).
The steps in the action cycle surrounding the knowledge creation funnel were derived from a theory analysis of planned action theories (Graham, Logan, Harrison, Tetroe, & the KT Theories Research Group, 2007). In this analysis, the researchers searched for planned action models—those specifically designed to be used to bring about change—in order to explore the theoretical underpinnings of knowledge translation. They excluded classical implementation theories, because, by definition, they are passive and used to retrospectively understand change. One example of a planned action theory/ framework is the Ottawa Model of Research Use (OMRU) (Figure 2), which focuses on moving research findings/evidence into practice (Graham & Logan, 2004). There are other good models—the researchers identified 31 in all (see database at http://www.iceberg-grebeci.ohri.ca/research/ kt_theories_db.html).
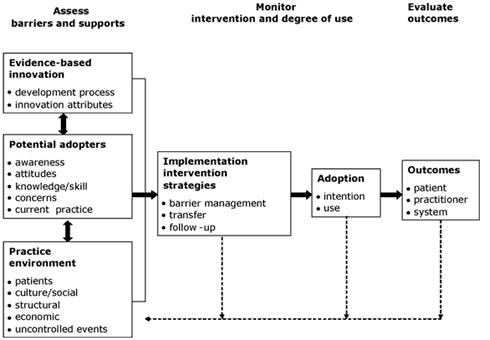
Figure 2. The Ottawa Model of Research Use
(Reprinted with permission from Canadian Journal of Nursing Research, Vol. 36, Graham, I. D. & Logan, J., Innovations in knowledge transfer and continuity of care, pp. 89–103, copyright © 2004.
The following 15 action categories were derived from sorting all of the constructs from all the planned action theories reviewed. A checkmark after the category indicates that it was covered in the OMRU.
- Identify the problem √
- Identify the need for change √
- Identify change agents √
- Identify target audience √
- Assess barriers √
- Review evidence/literature or develop innovation √
- Tailor/develop intervention √
- Link(age)
- Implement √
- Evaluate
- Develop evaluation plan
- Pilot-test
- Evaluate the process √
- Evaluate outcomes √
- Maintain change √
- Disseminate
These 15 action categories were further distilled into the seven boxes surrounding the knowledge funnel in Figure 1. We have found this conceptualization of the knowledge to action process to be a helpful and comprehensible tool for illustrating and explaining what we mean by KT to all of our stakeholders.
Knowledge Translation—Good in Theory, but How Can It Work in Practice?
Determining who is responsible for what in the knowledge translation process depends on the specific circumstances under consideration. The key process to keep in mind is linkage/communication with key research/knowledge users. In cases where implementation (knowledge to action) is required, it is critical to ensure agreement from all players on the need for change; the nature of the evidence for change; methods and the evaluation of the impact of the change; and who is responsible for each of the components.
Researchers interested in increasing the impact of their work through contributing to the KT process have a variety of options to pursue. They can contribute to both the science and the practice of KT; conduct needs assessments; conduct systematic reviews pointing to a need for change or to the knowledge that needs to be translated; create an appetite for research results; keep communication lines open; conduct research with a ready audience having a perceived need for the research results; be knowledge brokers; and be as systematic and rigorous as they would "normally."
How to Measure the Impact of Knowledge Translation
Measuring and attributing impact is difficult and still in its early stages within the health research field. How one would go about it depends on one's definition of impact as well as on the perspective of the knowledge user. For example, one could develop or adapt measures of each step of the knowledge to action cycle described in Figure 1, but there are no empirical data to suggest how to weigh the various components or how to attribute the presence or absence of any of those components to the degree of impact. Furthermore, there are different degrees of impact to consider. Beyer (1997) distinguishes between instrumental (applying research results in specific, direct ways), conceptual (using research results for general enlightenment; results influence actions but more indirectly and less specifically than in instrumental use), and symbolic (using research results to legitimize and sustain predetermined positions).
The Canadian Health Services Research Foundation (CHSRF) and the Alberta Heritage Foundation for Medical Research (AHFMR) have been making some progress on the question of assessing impact, as has CIHR. At CIHR (2005), we are working on a continually evolving framework for evaluation of the impact of the research we fund. An updated version of this framework has been published in the proceedings book for the Organisation for Economic Co-operation and Development Blue Sky II Forum (www.oecd.org/document/12/0,3343,en_2649_20 1185_39369868_1_1_1_1,00.html#TOC accessed October 1, 2007). Examples of sources of data/tools for measuring the impact of knowledge translation efforts include (but are not restricted to) citation impact analyses (e.g., bibliometric studies); endof- grant reports; case studies; interviews/surveys; administrative databases; and document analysis.
Making Sense of KT at CIHR
At CIHR, we have divided KT into two main categories: end-of-grant and integrated. With end-of-grant KT, the researcher develops and implements a plan for making users aware of the knowledge that has been gained from the project; in integrated KT stakeholders or potential research users are engaged in the entire research process.
By end-of-grant KT, we mean the typical dissemination and communication activities undertaken by most researchers: KT to their peers, such as through conference presentations and publications in peerreviewed journals. End-of-grant KT can also involve more intensive dissemination activities that tailor the message and medium to a specific audience, such as summary briefings to stakeholders; more interactive approaches such as educational sessions with patients, practitioners, and/or policymakers; media engagement; or the use of knowledge brokers. The commercialization of scientific discoveries is another form of end-of-grant KT.
The term integrated KT describes a different way of doing research with researchers and research users working together to shape the research process— collaborating on setting the research questions, deciding the methodology, being involved in data collection and tools development, interpreting the findings, and helping disseminate the research results. This approach, also known by such terms as collaborative research, participatory action research, action-oriented research, and co-production of knowledge, should produce research findings that are more likely be relevant to and used by the end users.
The expectation is not that every researcher be involved in integrated KT. However, the expectation is that research results are disseminated to the appropriate audience (which is often other researchers). Generally, the intensity of knowledge translation should depend on factors such as the potential importance/impact of applying the findings; the amount and strength of the evidence supporting the findings (often determined by synthesis); the target audience(s); what is known about effective strategies to reach the audience(s); and what is practical, ethical, and feasible to do under the circumstances.
CIHR has mechanisms to fund synthesis, end-of-grant KT, integrated KT, and the science of KT and is developing a number of policies and procedures to facilitate KT.
Table 1. Funding Opportunities
| Funding Opportunities | |
|---|---|
| KT Focus | Funding Mechanisms |
| Synthesis |
|
| Integrated KT |
|
| End-of-Grant KT |
|
| Science of KT |
|
Research Reporting System
The CIHR Evaluation and Analysis Branch, at the time this report was written, was developing a Research Reporting System (RRS). Before this, CIHR had no systematic method to collect, synthesize,
and report on research results, outputs, and potential impacts. Asking grant recipients to provide such information, which is common practice internationally, will allow us to better meet the knowledge
translation component of our mandate. Information will be collected in the following categories: nominated principal investigator (NPI) profile; basic grant information; research and KT practices;
research results; research capacity and training; advancing knowledge; and longer term impacts.
Open-Access Policy Implementation
CIHR recently unveiled a new policy to promote public access to the results of research it has funded. CIHR will require its researchers to ensure that their original research articles are freely
available online within 6 months of publication. Under this new policy, which will apply to all grants awarded after January 1, 2008, that receive funding in whole or in part from CIHR, grant recipients
must make every effort to ensure that their peer-reviewed research articles are freely available as soon as possible after publication. This can be achieved by depositing the article in an archive,
such as PubMed Central or an institutional repository, or by publishing results in an open-access journal. A growing number of journals already meet open-access requirements, and CIHRfunded researchers
are encouraged to consider publishing in these journals.
Additionally, grant recipients are now required to deposit bioinformatics, atomic, and molecular coordinate data, as already required by most journals, into the appropriate data repository.
Assessment of KT in Grant Applications (KT Assessor Project)
Through a contract funded jointly by CHSRF, CIHR, the Netherlands Organization for Health Research and Development (ZonMw), and the UK National Health Services Delivery and Organization Research
and Development Programme (SDO), Paula Goering and her team have provided a conceptual framework for guiding researchers and peer reviewers in developing/considering the most effective KT plan
for a particular research context (Goering, Ross, Jacobson, & Butterill, 2007). Based on this report, we are in the process of developing guidelines for both peer/merit reviewers and CIHR grant
applicants. The framework, while inclusive, will be scaled down to be of practical use for the majority of proposals submitted to CIHR.
Conclusion
Under the leadership of Dr. Ian Graham, and with the support of the executive team and the KT Portfolio staff at CIHR, the meaning of the process of knowledge translation—knowledge to action—is
becoming clarified, operationalized, and "un-scarified."
References
Beyer, J. M. (1997). Research utilization: Bridging the gap between communities. Journal of Management Inquiry, 6(1), 17–22.
Brown, S. J. (1999). Knowledge for health care practice: A guide to using research evidence. Philadelphia: W.B. Saunders.
The Canadian Institutes of Health Research. (2005). Developing a CIHR framework to measure the impact of health research (CIHR synthesis report). Retrieved September 7, 2007, from http://www.cihr-irsc.gc.ca/e/30324.html
Estabrooks, C. A. (1998). Will evidence-based nursing practice make practice perfect? Canadian Journal of Nursing Research, 30(1), 15–36.
Estabrooks, C. A. (2003). Translating research into practice: Implications for organizations and administrators. Canadian Journal of Nursing Research, 35(3), 53–68.
Estabrooks, C. A. (2006). Glossary: Knowledge Utilization Studies Program. Retrieved September 7, 2007, from http://www.kusp.ualberta.ca/Resources/Glossary.aspx
Goering, P., Ross, S., Jacobson, N., & Butterill, D. (2007). Towards more effective peer review of knowledge translation (KT) plans in research grant proposals (Unpublished manuscript).
Graham, I. D., & Logan, J. (2004). Innovations in knowledge transfer and continuity of care. Canadian Journal of Nursing Research, 36(2), 89–103.
Graham, I. D., Logan, J., Harrison, M., Straus, S., Tetroe, J. M., Caswell, W., & Robinson, N. (2006). Lost in translation: Time for a map? Journal of Continuing Education in the Health Professions, 26, 13–24.
Graham, I., Logan, J., Harrison, M., Tetroe, J., & the KT Theories Research Group. (2007). Planned action models/theories to implement evidence-based care: A review. Implementation Science (Under review).
Graham, I., & Tetroe, J. (2007). How to translate health researchknowledge into effective healthcare action. Healthcare Quarterly, 10(3), pg 21–23. Retrieved September 7, 2007, from http://www.longwoods.com/product.php?productid=18919&cat =492&page=1
Lomas, J., Culyer, T., McCutcheon, C., & McAuley, L. (2005). Conceptualizing and combining evidence for health system guidance. (Final report for the Task Force on Wait Times, Provincial/Territorial Conference of Deputy Ministers of Health). Retrieved September 7, 2007, from http://www.chsrf.ca/other_documents/evidence_e.php
Titler, M. G., Mentes, J. C., Rakel, B. A., Abbott, L., & Baumler, S. (1999). From book to bedside: Putting evidence to use in the care of the elderly. The Joint Commission Journal on Quality Improvement, 25(10), 545–556.
Last Updated: Tuesday, 23 July 2024 at 07:40 PM CST